Women's Health
Clinical Trials With Immunotherapy Drugs Are Source Of Hope And Challenges In Treating Aggressive Breast Cancer
|
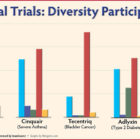
Joshalyn Mills of Branford and Nancy Witz of Kensington had the best possible results after being treated in clinical trials with immunotherapy drugs for aggressive breast cancer: Their tumors were eliminated. But while there are dramatic successes with immunotherapy drugs, there are also many failures, and researchers are trying to find out why in hopes of expanding the drugs’ effectiveness. Cutting-edge immunotherapy drugs use a person’s own immune system to fight disease. The Food and Drug Administration (FDA) first approved the drugs in 2011 for cancer treatment. Success has occurred in about 15% to 20% of patients with cancers such as melanoma, lung, kidney and bladder, according to a report by Johns Hopkins School of Medicine.